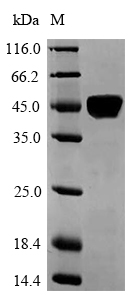
Laminin subunit alpha-5 (LAMA5) is a vital protein involved in various biological processes. Laminins are a family of proteins that constitute a significant part of basement membranes, crucial for maintaining tissue integrity [1]. LAMA5 acts as a specific receptor for laminin-511 (LM-511), a key component of basement membranes [2]. Research has demonstrated that LAMA5 is essential for regulating dendritic spine structure and ensuring synapse stability in the brain [3]. In mammary glands, the expression of LAMA5 in luminal epithelial cells is necessary for normal growth and development [4]. Additionally, LAMA5 is associated with preserving the integrity of the intestinal basement membrane, emphasizing its role in gastrointestinal health [5].
Moreover, LAMA5 is implicated in various diseases and conditions. It has been linked to promoting tumor cell migration in cancers such as ovarian cancer and colorectal liver metastasis [6][7]. Mutations in the LAMA5 gene have been connected to conditions like infantile nephrotic syndrome and skeletal dysplasia [8][9]. Furthermore, LAMA5 contributes to hair morphogenesis and tooth development [10][11].
Furthermore, LAMA5 is critical for kidney glomerular basement membrane assembly, and its deficiency can lead to conditions like polycystic kidney disease [12][13]. The protein also interacts with other molecules such as integrins and dystroglycans to facilitate basement membrane assembly and tissue polarization [14].
References:
[1] L. Jones, R. Lam, K. McKee, M. Aleksandrova, J. Dowling, S. Alexanderet al., "A mutation affecting laminin alpha 5 polymerisation gives rise to a syndromic developmental disorder", Development, 2020. https://doi.org/10.1242/dev.189183
[2] Y. Kikkawa, T. Ogawa, R. Sudo, Y. Yamada, F. Katagiri, K. Hozumiet al., "The lutheran/basal cell adhesion molecule promotes tumor cell migration by modulating integrin-mediated cell attachment to laminin-511 protein", Journal of Biological Chemistry, vol. 288, no. 43, p. 30990-31001, 2013. https://doi.org/10.1074/jbc.m113.486456
[3] S. Luo, Z. Liu, J. Wang, J. Luo, X. Ye, X. Liet al., "Recessive lama5 variants associated with partial epilepsy and spasms in infancy", Frontiers in Molecular Neuroscience, vol. 15, 2022. https://doi.org/10.3389/fnmol.2022.825390
[4] J. Englund, A. Ritchie, L. Blaas, H. Cojoc, N. Pentinmikko, J. Döhlaet al., "Laminin alpha 5 regulates mammary gland remodeling through luminal cell differentiation and wnt4-mediated epithelial crosstalk", Development, vol. 148, no. 12, 2021. https://doi.org/10.1242/dev.199281
[5] Y. Park, E. Ha, K. Gu, G. Shin, C. Lee, K. Kimet al., "Host genetic and gut microbial signatures in familial inflammatory bowel disease", Clinical and Translational Gastroenterology, vol. 11, no. 7, p. e00213, 2020. https://doi.org/10.14309/ctg.0000000000000213
[6] S. Sivakumar, S. Lieber, D. Librizzi, C. Keber, L. Sommerfeld, F. Finkernagelet al., "Basal cell adhesion molecule promotes metastasis‐associated processes in ovarian cancer", Clinical and Translational Medicine, vol. 13, no. 1, 2023. https://doi.org/10.1002/ctm2.1176
[7] A. Gordon-Weeks, S. Lim, A. Yuzhalin, S. Lucotti, J. Vermeer, K. Joneset al., "Tumour-derived laminin α5 (lama5) promotes colorectal liver metastasis growth, branching angiogenesis and notch pathway inhibition", Cancers, vol. 11, no. 5, p. 630, 2019. https://doi.org/10.3390/cancers11050630
[8] Y. Taniguchi, C. Nagano, K. Sekiguchi, N. Sato, H. Sakaguchi, C. Umedaet al., "Clear evidence of lama5 gene biallelic truncating variants causing infantile nephrotic syndrome", Kidney360, vol. 2, no. 12, p. 1968-1978, 2021. https://doi.org/10.34067/kid.0004952021
[9] M. Barad, F. Csukasi, M. Bosakova, J. Martín, W. Zhang, S. Tayloret al., "Biallelic mutations in lama5 disrupts a skeletal noncanonical focal adhesion pathway and produces a distinct bent bone dysplasia", Ebiomedicine, vol. 62, p. 103075, 2020. https://doi.org/10.1016/j.ebiom.2020.103075
[10] S. Fukumoto, J. Miner, H. Ida, E. Fukumoto, K. Yuasa, H. Miyazakiet al., "Laminin α5 is required for dental epithelium growth and polarity and the development of tooth bud and shape", Journal of Biological Chemistry, vol. 281, no. 8, p. 5008-5016, 2006. https://doi.org/10.1074/jbc.m509295200
[11] J. Gao, M. DeRouen, C. Chen, M. Nguyen, N. Ngôn, H. Idoet al., "Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis", Genes & Development, vol. 22, no. 15, p. 2111-2124, 2008. https://doi.org/10.1101/gad.1689908
[12] M. Shannon, B. Patton, S. Harvey, & J. Miner, "A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease", Journal of the American Society of Nephrology, vol. 17, no. 7, p. 1913-1922, 2006. https://doi.org/10.1681/asn.2005121298
[13] B. Steenhard, A. Zelenchuk, L. Stroganova, K. Isom, P. John, G. Andrewset al., "Transgenic expression of human lama5 suppresses murine lama5 mrna and laminin α5 protein deposition", Plos One, vol. 6, no. 9, p. e23926, 2011. https://doi.org/10.1371/journal.pone.0023926
[14] S. Li, Y. Qi, K. McKee, J. Liu, J. Hsu, & P. Yurchenco, "Integrin and dystroglycan compensate each other to mediate laminin-dependent basement membrane assembly and epiblast polarization", Matrix Biology, vol. 57-58, p. 272-284, 2017. https://doi.org/10.1016/j.matbio.2016.07.005